Dr Musa Mohd Nordin, Paediatrician
Dr Tan Hui Siu, Paediatrician
The AstraZeneca (AZ) COVID-19 vaccine has been in the news lately.
The press reportings has not been investigative and precise enough, let alone fair, giving rise to anti-vaccine proponents thinking they have been right all along. One anti-vaccine doctor was bold enough to suggest that other COVID-19 vaccine manufacturers would eventually come clean with their safety data and withdraw all their vaccines — the same one who claimed that “…this pandemic is the greatest hoax of human history.” (1,2)
Any investigative reporting would have considered in detail the benefits and risks of the AZ COVID-19 vaccine to individuals and the role in saving most lives in a larger context of the then-raging pandemic.
The story of the AZ covid vaccine begins with how the vaccine experts at the Jenner Institute, University of Oxford, were futuristically designing a vaccine model to defeat an unknown pandemic pathogen. They infected chimpanzees with the common cold virus (adenovirus), and re-engineered it to be the backbone of a vaccine against any pathogen. They named it Chimpanzee Adenovirus Oxford One, or ChAdOx1. (3)
When Chinese scientists shared the complete genome of SARS-CoV-2 on 11 January 2020, the Oxford team inserted the genetic code of the spike protein into ChAdOx1 and developed a vaccine against SARS-CoV-2 in a record time of 10 months.
Phase 3 ChAdOx1 COVID-19 vaccine randomised control trials demonstrated a vaccine efficacy of 76%, which increased to 82% with a second dose 12 weeks later. (4)
In just a year, in January 2021, the United Kingdom, with the second highest COVID-19 death toll in Europe, became the first country to roll out this vaccine, co-developed by the University of Oxford and AstraZeneca. (5)
A month later, WHO approved the Oxford/AstraZeneca COVID-19 vaccine for emergency use, and vaccines were rolled out globally through COVAX (6).
The vaccine was one of the most affordable ones in the market – almost five to ten times cheaper than the mRNA vaccines. It would have been interesting to know the exact price tags in various countries, including Malaysia.
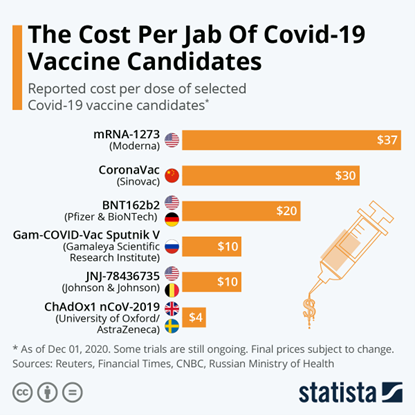
Apart from being affordable, the AZ COVID-19 vaccines were easier to transport, distribute, and administer since it did not require storage at arctic low temperatures.
The rare but serious adverse effects of AZ COVID-19 vaccine were known as Thrombosis Thrombocytopenia Syndrome (TTS), which is the presence of blood clots (thrombosis) developing in unusual locations, such as the cerebral veins, and having low platelet levels (thrombocytopenia).
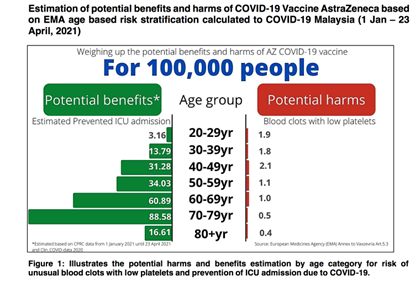
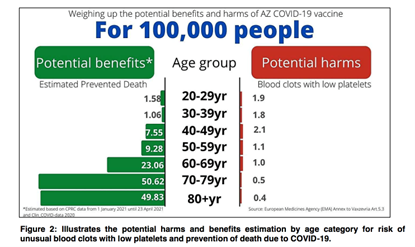
The Adverse Effects Following Immunisation (AEFI) monitoring and pharmacovigilance data showed that in those aged less than 70, the rates of hospitalisation due to TTS were higher after the vaccination compared to those unvaccinated and those aged above 70 (7).
However, in general, the risks of venous thrombosis, thrombocytopenia, and cerebral venous thrombosis in COVID-19 infection had been quoted as 190, 9, and 3 times higher than those associated with the vaccination. (8)
With the emergence of this data, the indication of AZ COVID-19 in many countries were limited to elderly persons, but not for Malaysia.
The Ministry of Health and JKJAV of Malaysia sanctioned the voluntary uptake of the AZ COVID-19 vaccines among younger persons. (9)
The then minister of health stated, “Science and facts could not overcome the public’s fear (over the AZ COVID-19 vaccine) because we understand that this is what the public feels.” (10)
Some of us raised the red flag in 2021, asking how MOH could scientifically and ethically justify AZ COVID-19 vaccine to younger adults when Britain, and later Europe, with the most extensive AZ COVID-19 vaccine experience, had advised otherwise? (11)
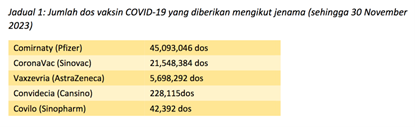
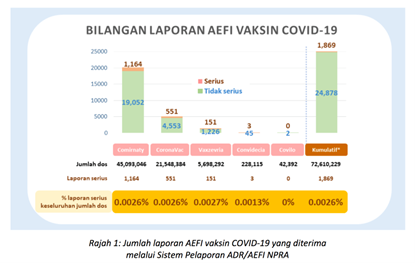
5,698,292 (7.8%) doses of AZ COVID-19 vaccines (Vaxzevria) were utilized up until 30 November 2023 in Malaysia (12) with 151 (0.0027%) reports of severe AEFIs following the vaccine. Out of this number, up to 31 March 2024, NPRA reported five cases of TTS or 0.88 cases per one million of doses of AZ COVID-19 vaccine given. (13). Comparatively, the European Medicines Agency (EMA) reported a rate of 5 cases per million in those above the age of 70 and 20 per million in those below the age of 50 years. (11)
An Imperial College London study calculated that COVID-19 vaccines saved 20 million lives between December 2020 and December 2021. Airfinity’s further analysis of this data showed that the Oxford/AZ COVID-19 vaccine saved 6.3 million lives, Pfizer/BioNTech 5.9 million lives, Sinovac 2 million lives and Moderna 1.7 million lives.
The mRNA COVID-19 vaccines were more robust, easier, and faster to update than AZ COVID-19 vaccines. Considering the “surplus of updated vaccines,” AstraZeneca has recently initiated the worldwide withdrawal of its COVID-19 vaccine.
Notwithstanding the pending court cases against AZ, one must not dismiss the role Oxford/AZ COVID-19 vaccine played in the early days of the pandemic in saving many lives.
The benefits of vaccination in preventing severe COVID-19 disease and deaths outweighed the risks of TTP if considered independently and in the proper age category. The efficacy and safety data of the AZ COVID-19 vaccine were published from the start. It was up to each country to critically appraise the scientific evidence and tailor the roll-out according to emergency needs and the benefits and risks calculation of different populations.
REFERENCES:
- https://www.nst.com.my/world/world/2024/05/1047661/astrazeneca-says-it-will-withdraw-covid-19-vaccine-globally-demand-dips
- https://m.malaysiakini.com/news/563833
- https://www.bbc.com/news/health-55040635
- https://www.sciencedirect.com/science/article/pii/S0140673621004323
- https://www.weforum.org/agenda/2021/01/uk-astrazeneca-vaccine-covid19/
- https://www.who.int/news/item/15-02-2021-who-lists-two-additional-covid-19-vaccines-for-emergency-use-and-covax-roll-out
- https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1003926
- https://www.bmj.com/content/374/bmj.n1931
- https://covid-19.moh.gov.my/garis-panduan/garis-panduan-kkm/Annex_48b_CLINICAL_GUIDELINES_ON_COVID-19_VACCINATION_AstraZeneca_2802021.pdf
- https://www.theedgemarkets.com/article/astrazenecca-vaccine-dropped-covid19-immunisation-programme-says-khairy-jamaluddin
- https://drmusanordin.com/2021/05/05/is-the-first-come-first-served-vaccine-rollout-scientifically-and-ethically-right/
- https://www.npra.gov.my/easyarticles/images/users/1066/AEFI%20Summary/Laporan-Ringkasan-Mengenai-AEFI-Vaksin-COVID-19_V8-data-sehingga-20231130.pdf
- https://codeblue.galencentre.org/2024/05/02/dr-dzul-wants-astrazenecas-explanation-on-covid-19-vaccine-side-effects/
- https://www.economist.com/graphic-detail/2022/07/13/which-covid-19-vaccine-saved-the-most-lives-in-2021



